A FEW years in the past, molecular biologists made an enormous breakthrough. By borrowing an antiviral mechanism from bacteria, they created a simple method to tweak the genetic data inside cells – a method that had huge implications for science, medicine & agriculture. However, a dispute over who precisely invented what first has threatened to curtail its potential. But CRISPR-Cas9, as the disputed technique is known as, turns out to not be the only means bacteria defend themselves from the attentions of viruses.Recently, in Cell, a group of researchers led by Feng Zhang of the Broad Institute in Cambridge, Massachusetts, who is among the parties to the patent dispute, have announced their discovery of another such mechanism.
Like its predecessor, the new mechanism, called CRISPR-Cpf1, has the potential to turn into a tool that may deal with intractable genetic diseases such as Huntington’s, and degenerative conditions like Alzheimer’s. It may also be used to create new classes of anti-viral treatment, and thus curb infectious disease. Plant and animal breeders, as well as doctors, might find it helpful, too. They could create new strains of crops and livestock. Indeed, because, like CRISPR-Cas9, it doesn’t involve taking genes from one organism and implanting them in another, it is not going to count as “transgenic”, a bogeyman of campaigners and customers alike.
CRISPR-Cas9 consists of a molecular pair of scissors (the Cas9) and a guide sequence of DNA (the CRISPR) that tells the scissors where to chop. The dispute over who can rightfully claim inventorship has prompted some, although not all, potential companies to give the technology a wide berth. Monsanto, a plant-breeding and agrichemicals agency, has gone on record as saying that it’s reluctant to make use of CRISPR-Cas9 widely till it understands the intellectual property concerned. Drug corporations have also circled at a distance. CRISPR-Cpf1, which has a different pair of scissors, might effectively not endure these legal issues.
CRISPR-Cpf1 can also be better than CRISPR-Cas9 in other ways. Cpf1 is a smaller and less complicated enzyme (identified technically as an endonuclease) than Cas9, which suggests it is going to be simpler to deliver to the cells whose genes need modifying. And its slightly offset cuts to double-stranded DNA will help researchers to insert genetic patches more effectively and precisely.
Its discovery also raises the question of how many other endonuclease-based systems are out there in the world’s bacteria. Viral infection is a serious threat to these microbes, and the natural job of both CRISPR-Cas9 and CRISPR-Cpf1 is to recognise viral genes and chop them up before they can do any harm. Conversely, viruses are constantly evolving to escape the antiviral systems’ attentions, meaning bacteria need to generate new ones. The chances are good, therefore, that CRISPR-Cas9 and CRISPR-Cpf1 are not alone. As Dr Zhang himself puts it, “I can’t even begin to count how many there may be. There really is great diversity that we as a scientific community should go out and explore.”
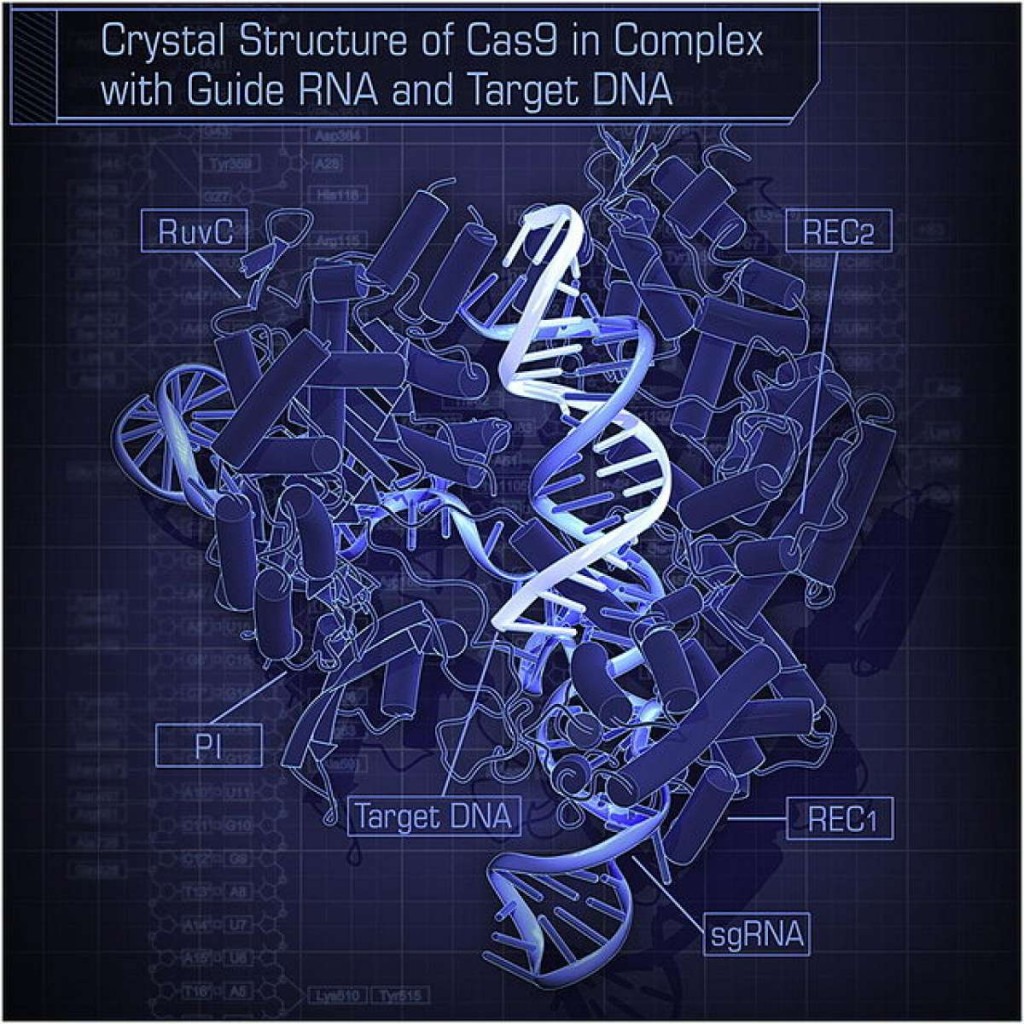
The tools to carry out that exploration now exist. CRISPR-Cpf1, for instance, was found not by searching in bacteria directly, but by scrutinising a published database of bacterial genetic sequences, which yielded two species that contain it. Further searches might be equally rewarding—and the more gene-editing systems are discovered, the harder it will be to monopolise their use.
Despite the optimism of those who think the new techniques may calm qualms about genetic engineering, however, some people are bound to have ethical worries—certainly when it comes to applying them to human beings. Earlier this year, for example, when Chinese scientists used CRISPR-Cas9 gene editing on a human embryo (albeit one that was unviable, and could not therefore have developed into a person) there was much brouhaha and several calls for a moratorium on this line of inquiry.
In December, therefore, a group of academic societies from America, Britain and China are hosting a meeting in Washington, DC, to thrash out some of the issues—particularly about the way in which gene editing might be used to make changes in human beings that could be inherited. On September 18th researchers in Britain applied for permission to modify human embryos genetically, as part of a study of human development. Any licence would allow the use only of embryos left over after IVF treatment, and those embryos could be studied for no more than two weeks of development. Nor could they be used to create a pregnancy, or for any other clinical application. But the direction of travel is clear.
The Washington meeting is thus timely. Scientists need to set out to politicians, and to the public at large, what gene-editing technology can now do. They could also speculate usefully about what it might mean for the future, and how best to use it. Dr Zhang is one of those who will attend. He will say that it is the responsibility of scientists and developers of the techniques to make sure that they engage the public in thoughtful discussion, and that the technology is used to best advantage.
This is a responsible attitude, and reminiscent of a meeting that happened 40 years ago in Asilomar, California, when early genetic engineers got together to explore the implications of their science. Then, they agreed a strict set of safety conditions and a self-imposed moratorium on some sorts of research that might have been dangerous, which helped to allay public fears. As more recent experience with genetically modified crops has shown, it is important to be up-front and open in this way. It would be a pity if CRISPR-based gene editing, having escaped the legal thickets, were to get tangled up in a PR disaster.










































[…] Source: Scientists have found a new way to edit genes – GadgTecs […]
[…] Source: Scientists have found a new way to edit genes – GadgTecs […]
[…] Source: Scientists have found a new way to edit genes – GadgTecs […]